A Triple Bond Is Generally Composed of
The two light green. A triple bond consists of one sigma bond and two pi bonds.
Illustrated Glossary Of Organic Chemistry Triple Bond
One eqpi eq bond and two eqsigma eq bonds.

. Three eqpi eq bonds b. The σ bond is made by a head-on overlap between two compatible atomic orbitals that are symmetric about the internuclear axis. A triple bond is generally composed of A one Δ bond and two Η bonds.
12 What is the geometry around the central atom in the following molecular model of BCl3. C three Η bonds. One of the electron pairs is present in a sigma bond concentrated in the region along the line joining the two nuclei.
Two eqpi eq bonds and one eqsigma eq bond c. The atoms with triple valency are pnictogen or the nitrogen family. 4 things around atom bond angle is 1095 degrees A triple bond is composed of sigma bond and two pi bonds.
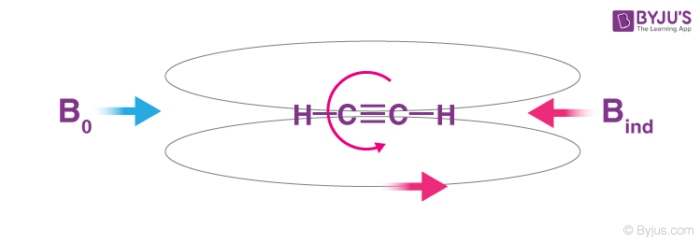
A triple bond is generally composed of. A triple bond is a type of bond formed between the two atoms that involve six bonding electrons in place of usual two in a single covalent bond. A triple bond consists of one sigma bond and two pi bonds.
The π bonds are made by the sidelong overlap between two compatible atomic orbitals. A triple bond consists of one sigma bond and two pi bonds. View the full answer.
Difference between Single Double and Triple Bond Chemical bonds are forces established between the electrons and nuclei of two atoms that hold atoms in a molecule together. B two Δ bonds and one Η bond. 1 Approved Answer Wahid A answered on February 16 2021 5 Ratings 14 Votes Answer.
Single bonds consist of one sigma σ bond double bonds have one σ and one pi π bond and. Together the two dark green bands are one pi bond. In chemistry a triple bond is defined as a covalent linkage where two atoms share three pairs of electrons as in the nitrogen molecule acetylene C2H2 or N2.
A triple bond is generally composed of A one Δ bond and two Η bonds. When three pairs of electrons are shared between two atoms then a triple bond is formed. D one π bond and two σ bonds.
It is generally composed of one σ -bond and two π -bonds. Therefore the correct option is b. Triple bond in chemistry a covalent linkage in which two atoms share three pairs of electrons as in the nitrogen molecule N 2 or acetylene C 2 H 2.
Co s Fe². In covalent bonddouble lines indicate a double bond between two atoms ie involving two electron pairs and triple lines represent a triple bond as found for example in carbon monoxide CO. The other two pairs are present in pi bonds each of which occupies two parallel regions of space on opposite sides of.
D three Δ bonds. Two pi bonds and one sigma bond. B two Δ bonds and one Η bond.
A triple bond is generally composed of A. D three Δ bonds. The carbon-carbon triple bond is made stronger by the presence of one sigma bond.
Triple bond is denoted by three dash joining the atoms. The image shows how they are arranged between two nitrogen atoms. It is generally composed of one σ -bond and two π -bonds.
One sigma bond and two pi bonds make up a triple bond in an alkyne. B three π bonds. Atoms a and c.
One bond and two. Two bonds and one. DO NOT COPY FROM CHEGG I NEED A FULL EXPLANATION Expert Answer Two pi bonds and one sigma bond.
The C-H distance in acetylene is 108 shorter than alkenes due to the fact that sp hybridized carbon has more s character than the sp 2 hybridized carbon. Chemical reactions are controlled by the formation or dissolution of chemical bonds. Pyrolysis of Organic Molecules Second Edition 2019.
YOU MIGHT ALSO LIKE. C three Η bonds. Two common examples are ss and spz combinations where lets say the z axis is the horizontal internuclear axis.
The main difference between single double and triple bond is the number of shared electrons. In the drawing of acetic acid CH3CO2H a partial positive charge 6 occurs on A. A triple bond is a type of bond formed between the two atoms that involve six bonding electrons in place of usual two in a single covalent bond.
C Because according to valence bond theory a triple bond is actually composed of two different kinds of bonds one σ and two π Advertisement New questions in Chemistry Predict whether the following reaction would proceed spontaneously as written at 298 K given that Co² 015 M and Fe² 068 M. The remaining two pairs are available in pi bonds each of which occupies the two parallel. 18 terms Orgo Exam 3 Chapter 7 Vocab 51 terms CEM 143 EXAM 1 36 terms Chemistry 14D Final Vocabulary 14 terms Chapter 9 OTHER SETS BY THIS CREATOR 43 terms Final exam concepts PHYSICS 10 terms.
The triple bond is formed from a σ bond between the sp orbitals of the two carbons with sp hybridization and two π bonds between the py and pz orbitals of the two carbon atoms. The two carbon atoms of the triple bond and the two atoms direct each other due to the shape of the sp hybrid orbitals. Only atom a B.
A triple bond is generally composed of A. C two π bonds and one σ bond. Triple bonds are formed by sharing three pairs six atoms of electrons.
A triple bond is generally composed of a three σ bonds. Acetylene is a linear molecule with carbon-carbon distance of 121A o. If the shared number is one pair of electrons the bond will be a single bond whereas if two atoms bonded by two pairs four electrons it will form a double bond.
Therefore the correct option is b. Only atom b C. One of the main electron pairs exists in a sigma bond which is concentrated in the region along the line joining the two nuclei.

Degrees Of Unsaturation Or Ihd Index Of Hydrogen Deficiency Index Chemistry Organic Chemistry

Triple Bond Examples Studiousguy

Balloon Drawing Triple Bond Between 2 Carbons Pi Bond Chemistry Bond
Comments
Post a Comment